In the dynamic landscape of biotechnology, ICON’s recent survey of over 100 leaders from the biotech industry dissects the challenges and opportunities faced by biotech companies. The findings show that, against the backdrop of funding challenges and intricate clinical trial landscapes, drug development emerges as the linchpin, where resilience and innovation converge. This article explores strategies for accelerating this critical process, emphasising the potential of partnerships to strategically support biotech companies and the industry’s commitment to addressing unmet healthcare needs.
Strategies to accelerate biotech drug development
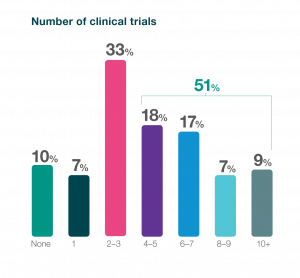
One of the key findings of the survey was that just under a third (32%) of respondents to the survey identified clinical trials as the most challenging stage in the drug development process. Despite this, biotechs identified an undertaking of 2-3 ongoing clinical trials as the most frequent range. When asked about the clinical trial challenges they face, respondents said clinical trials are extremely costly, require significant expertise and coordination; are the most time- consuming element; and it’s hard to find candidates willing to volunteer for clinical studies.
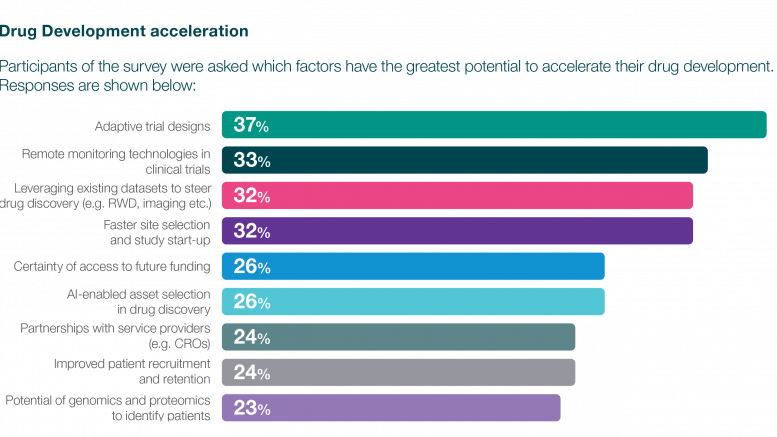
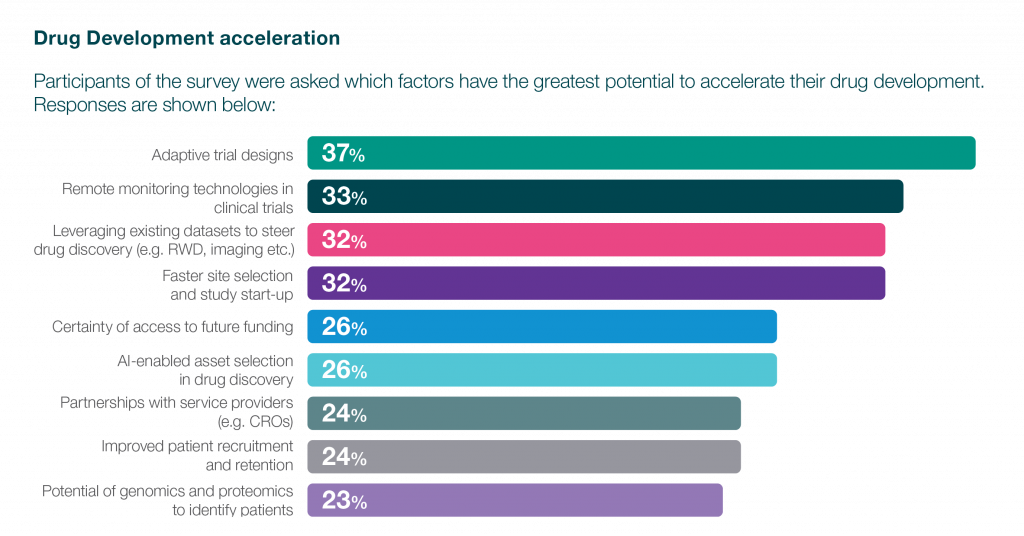
So, how can these challenges be overcome? The survey findings indicate that biotechnology is recognising the value in adopting transformative strategies and innovative technologies to accelerate clinical development programs. One aspect of this transformation lies in trial designs. Biotechs are embracing innovative trial designs that not only expedite the development timeline but also enhance decision-making processes. Adaptive methodologies, allowing adjustments based on interim data provide the flexibility to refine trial parameters as insights unfold, mitigating risks and optimising outcomes. While not a one-size-fits-all solution, adaptive and model- based trials can mitigate risks when carefully planned, despite their potential complexity.
Decentralised trials leverage technology to streamline processes, reduce costs, and enhance patient engagement, ultimately contributing to shorter development timelines.
Similarly, the role of data analytics, artificial intelligence (AI), and machine learning (ML) is paramount in this landscape. These technological advancements empower biotechs with the tools to extract meaningful insights from vast datasets. By discerning patterns, predicting outcomes, and optimising trial designs, AI and ML foster a level of informed decision-making that was once unimaginable. The marriage of data analytics with clinical expertise not only expedites decision-making but also enhances the overall efficiency and efficacy of drug development programs.
Significance for investors
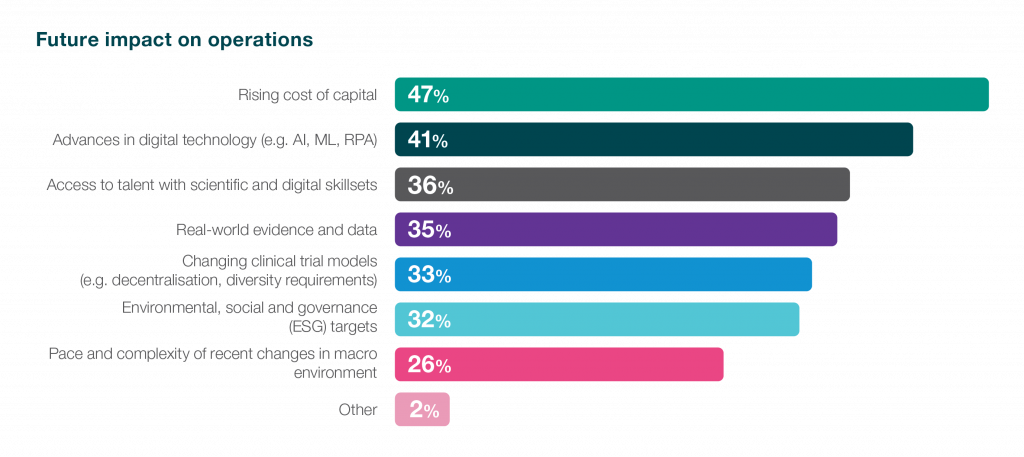
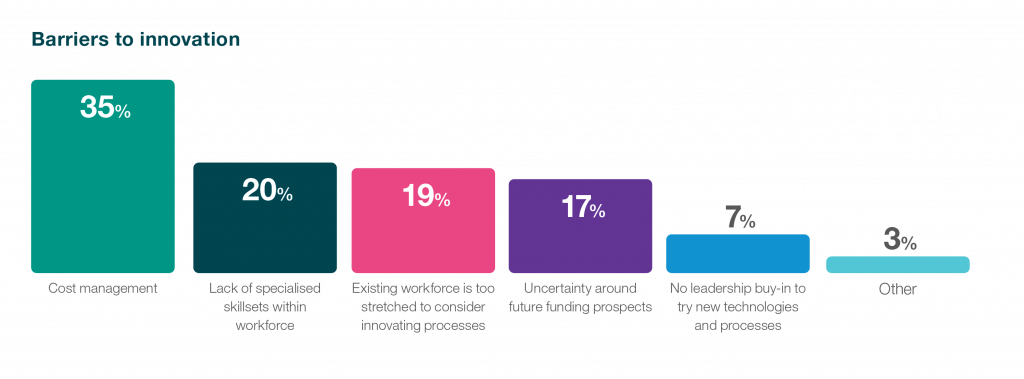
Securing funding is a perennial priority for biotech companies. 47% of survey respondents highlighted the influence of the escalating cost of capital on future operations, while 35% identified cost management as the primary obstacle to fostering innovation. These statistics shed light on the financial tightrope that biotech organisations navigate, with the rising cost of capital presenting a hurdle for future endeavours. As the industry grapples with these challenges, strategic partnerships, innovative funding models, and operational efficiencies become pivotal in sustaining momentum and driving successful drug development initiatives. Navigating this financial landscape with resilience and ingenuity is not only important for the immediate health of individual biotech organisations but also crucial for instilling confidence in potential investors. In a sector where ground-breaking advancements demand substantial financial backing, the industry’s ability to address these funding challenges directly influences investor confidence and, consequently, the trajectory of biotech innovation. Innovative discoveries often face lengthy and complex journeys from concept to market, and the ability to navigate this path efficiently becomes a key determinant of investor trust and support.
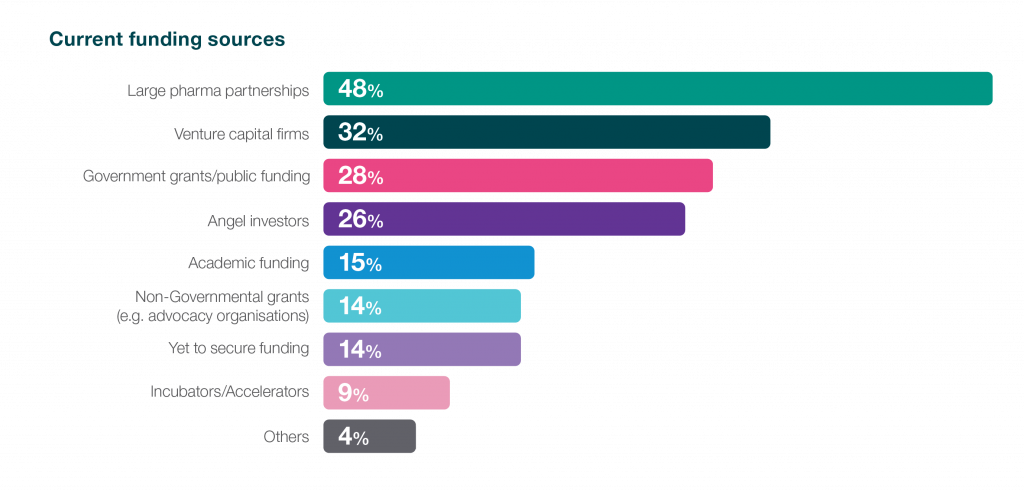
The survey findings highlight that 48% of respondents currently rely on partnerships with large pharmaceuticals for funding, with 32% securing investments from venture capital firms. Respondents also explore public/non-profit funding and financial markets as alternative funding sources. In the dynamic biotech landscape, where securing financial backing is pivotal, fostering investor confidence becomes indispensable. The reliance on strategic partnerships and venture capital not only highlights the industry’s adaptability but also underscores the crucial role investor confidence plays in propelling biotech innovations.
Investors, keenly aware of the challenges intrinsic to clinical trials, are increasingly recognising the value of biotech organisations that demonstrate adeptness in executing their development programs. The efficiency and innovation with which a biotech manages its clinical trials directly impact the timeline and success of drug development. Investors understand that swift progress through clinical phases not only accelerates the time to market but also minimises the financial burden associated with prolonged development. Moreover, innovative execution strategies showcase a biotech’s capacity to adapt and optimise its approach in response to evolving circumstances. This adaptability is crucial in the dynamic landscape of drug development, where unforeseen challenges can arise. Biotechs that can effectively manage clinical trials with agility and foresight signal to investors a commitment to overcoming obstacles and a readiness to capitalise on opportunities.
Adaptive trial design offer significant potential to expedite drug development programs, in addition to capabilities such as remote monitoring, leveraging existing data, and efficient site selection. Recognising the critical need for these advanced strategies, biotech organisations are increasingly forging partnerships with service providers and pharmaceutical collaborators. The evolving clinical landscape has spurred new outsourcing models and operational strategies, propelled by a growing imperative for partnerships between biotechs and CROs.
This emphasis on collaboration extends beyond operational efficiency; it becomes a catalyst for streamlining trial designs across the entire drug development process. A partnership with a CRO extends its impact from the preclinical stages to the formulation of commercial strategies. For instance, the collaboration between a biotech company and a CRO becomes instrumental in integrating patient-centric approaches into trial designs and establishing networks with venture capital firms to secure essential clinical research funding. As the industry recognises the value of seamless execution in clinical trials, these collaborative efforts emerge not only as a response to challenges but as a proactive strategy to transform drug development programs.
Collaboration and early strategic engagement
In the intricate landscape of biotech development, the concept of wider industry support and engagement emerges as an important factor, underscoring the significance of early strategic engagement. Collaborating with various industry stakeholders—pharmaceutical companies, CROs and patient advocacy groups—presents a wealth of resources, expertise, and a collaborative environment essential for overcoming shared challenges.
While CROs play a pivotal role in supporting clinical trials, the limitations of ad hoc arrangements can impede the seamless progression of drug development. Recognising this, the emphasis shifts to the importance of cultivating long-term partnerships with CROs. Such alliances ensure continuity and integration, addressing the gaps that ad hoc arrangements may introduce. A consistent, mutually beneficial collaboration with CROs becomes a cornerstone, transcending individual projects and contributing to the sustained success of drug development programs.
This type of collaboration not only enriches the biotech landscape with shared expertise but also creates a resilient framework where challenges are collectively addressed, leading to more efficient and successful drug development endeavours.
While the industry is presented with a myriad of challenges, one thing is clear from the survey – biotech organisations will continue their mission of advancing life-changing treatments and addressing unmet medical needs, ultimately contributing to a brighter and more innovative future for the industry and its patients.
Dr. Chris Smyth, President, ICON Biotech
Dr. Smyth has 30 years’ operational and therapeutic experience, most notably in biotech, MedTech and oncology. Prior to ICON, he spent 10 years in IQVIA Biotech, where he held EVP Oncology and Chief Operating Officer roles before assuming global leadership as President of the division. Dr. Smyth also previously led global clinical operations for 8 years at an oncology biotech company, Antisoma. Dr. Smyth holds a Bachelor of Science degree in Biochemistry from the University of Kent at Canterbury, a PhD in Reproductive Biology from the University of Edinburgh Medical School, and an MBA from Henley Management College.