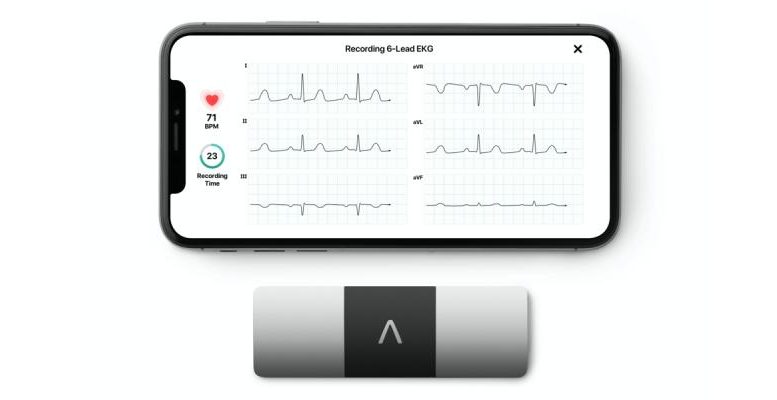
ERT has announced a first-of-its-kind partnership with AliveCor, the leader in AI-based, personal ECG technology, that will enable patient-administered ECG assessment for continuation of clinical trials during COVID-19. The partnership enables ERT to capture digital cardiac safety data with KardiaMobile 6L, the only FDA-cleared personal ECG for patient-administered 6-lead data collection.
ERT is a global data and technology company that minimizes uncertainty and risk in clinical trials so that its customers can move ahead with confidence.
“By combining AliveCor’s advanced technology with our proven software and workflow platform, we are enabling our customers to continue developing new medical treatments during the COVID-19 pandemic, regardless of whether trial patients have physical access to investigative site personnel,” said Ellen Street, Executive Vice President of Cardiac Safety of ERT. “The device’s ease of use, combined with ERT’s centralized over-read and data collection methodology make it an ideal solution for ensuring patient safety during ongoing clinical trials.”
KardiaMobile 6L is a hand-held, 6-lead personal ECG that records Lead II data without the attachment of electrodes. Data captured from the device will be integrated into ERT’s software and workflow platform and read by ERT cardiologists to ensure patient safety during the clinical development of new medical treatments. ERT provides high quality measurements for QTc, QRS, and other ECG intervals that enable efficacy and safety monitoring during clinical trials.
“ERT’s experience in analysing millions of ECGs and their dedication to patient safety make them the gold standard in clinical trial cardiac safety assessment,” said Priya Abani, CEO of AliveCor. “We look forward to this partnership and to delivering the innovative solutions and valuable data that clinical trial sponsors require.”