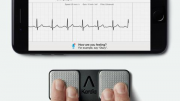
A hand-held device that would give early warning of ‘acute events’, and so avoid the hospitalisation of thousands of patients with chronic obstructive pulmonary disease (COPD), is to go into production ahead of clinical trials. This follows an agreement between medical diagnostics company Glyconics and Spectrolytic, a leading developer of infrared (IR) spectrometers.
IR spectrometry is proven as a non-invasive technique for diagnosis of a range conditions including COPD and diabetes, but the current IR technology is too large to be used outside of a hospital.
Cambridge-based start-up Glyconics is the first to miniaturise IR technology to enable a low-cost device suitable for use by healthcare professionals and their patients at home.
Dr Niall Gallen, CTO at Glyconics, says: “Our technology has gained proof of concept and we are now accelerating the development of our device and platform. By entering an exclusive agreement with Spectrolytic we will be able to produce Glyconics devices for testing ready for major clinical trials next year.”
Spectrolytic is the world leader in the use of spectroscopy for industrial and food applications; through the agreement with medical diagnostics specialists Glyconics it will produce the world’s first hand-held IR devices for COPD and other conditions.
Carsten Giebeler, CEO of Spectrolytic, comments: “Spectrolytic was founded with the aim of making spectroscopy solutions more widely available. Glyconics are recognised leaders in spectroscopy for medical areas; this expertise will enable us to produce a low cost device that can be used in the home.”
Professor Anoop Chauhan, Director of Research & Innovation at Portsmouth Hospitals NHS Trust, says the initial application for COPD is exciting:
“At the moment, when patients develop an exacerbation, they become very breathless and very wheezy, and as a result they require increased levels of treatment. Some patients have crisis exacerbations and they end up in hospital. These attacks are preventable if treatment is given early but we currently have no technology able to predict them. It is this unmet need that Glyconics is addressing.”
Earlier this year the European Commission awarded Glyconics a coveted Seal of Excellence, a quality label awarded to project proposals submitted to Horizon 2020 which succeeded a highly competitive evaluation process by independent experts. This quality label is a guarantee for investors to find high standard project proposals from European SMEs with growth potential. Glyconics was also identified as a ‘Top 50 Cambridge Companies: One to Watch’ by Business Weekly.
The Glyconics devices will be ready for user trials Q1 2019