The widespread availability of skin cancer self-assessment technology – apps designed to help patients identify potentially suspect skin lesions – has resulted in greater numbers of people seeking referrals for skin concerns.
In the UK, 24% of the population visit GPs each year with a skin problem. Around 1 million suspect skin lesions are referred to secondary care, 98% of which prove to be non-melanoma. This over-demand for scarce dermatology services results in substantial costs that have been estimated to be more than £250 million p.a. in the UK. In the USA, the annual costs of melanoma treatment in 2015 reached $3.3 billion of the total $8.1 billion in skin cancer costs.
With waiting lists increasingly becoming longer, there is a need for technology that can help primary care physicians triage patients with these conditions.
Here we speak to Joe Ferreira from British med-tech company, Moletest, a company that is developing the first and only ‘rule-out’ screening device – called nomela– for skin lesions suspected of melanoma skin cancer.
What is nomela?
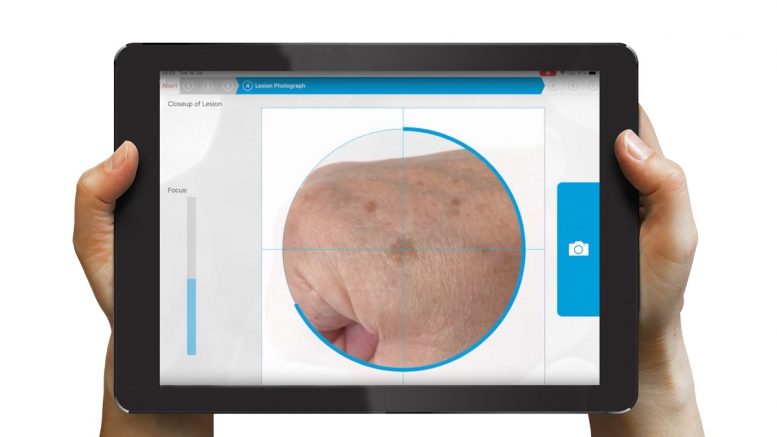
Nomela is a CE marked rule-out screening test for pigmented skin lesions suspected of melanoma. Using an iPad app, physicians can take high quality images from which they are then given a given an instant analysis of the lesion to aid patient management. A “Melanoma not excluded” result means further clinical assessment is advised. A “No evidence of melanoma” result which, in the absence of clinical suspicion, allows the patient to be reassured. nomela can also be used for image capture for later review without a report if there are contra-indications.
What challenges in primary care dermatology are you looking to solve with Nomela?
GPs are increasingly risk-averse when triaging skin lesions for a variety of reasons. These include having less time to see patients, awareness of clinical negligence claims allied to the fear of missing a diagnosis. This in turn, has contributed to an already overwhelmed referral pathway, so a rule-out medical device that can safely but significantly support the triage decision would have a major impact on reducing the referred numbers.
From your experience, how does the technology allow providers to optimise their approach towards skin cancer screening?
In a clinical trial, undertaken at NHS Lanarkshire, the nomela technology has demonstrated that it can safely remove over 50% of referrals from the pathway as ‘no evidence of melanoma’. By implementing the technology, it can result in the significantly optimised delivery of interventions and treatment leading to a much more manageable pathway.
We are looking to produce a clinical-grade decision support tool that can reliably support caregivers in their diagnoses. In a current ongoing clinical trial with Addenbrookes, in Cambridge, we are targeting a sensitivity figure of 95% +/- 2%.
How does this type of digital screening technology fit into the existing diagnostic and referral pathways?
Ideally, our technology would be used after primary care has eliminated the ‘confident decisions’ so it is left with the ‘I’m not sures’, or before secondary care. In the real world, the optimum setting is probably the intermediate/community services sector providing cases could be channelled through them appropriately.
Moletest is hoping to launch nomela, pending the successful completion of Clinical trial C8, which is expected to be completed sometime in early 2022.